
by Bryan Prince | Dec 28, 2021 | Uncategorized
One of the most common issues a cleanroom will encounter is inconsistent temperature and humidity parameters. Because of the varying weather conditions and atmospheric pressure outside the building, conditions inside the building also fluctuate. As a very dry and...

by Bryan Prince | Sep 21, 2021 | Uncategorized
No two cleanroom suites are designed alike. Effective PEC placement will differ for sterile non-hazardous compounding versus sterile hazardous compounding, for example. Further, some cleanrooms may have space constraints, presenting challenges for placing PECs, fan...
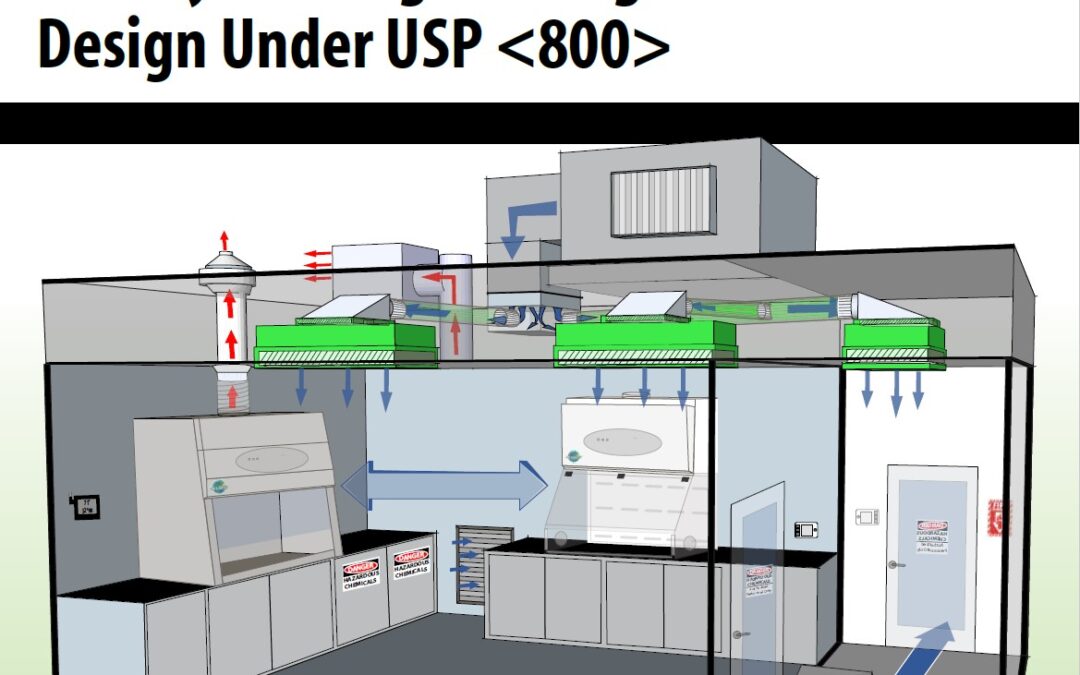
by Bryan Prince | Feb 16, 2021 | Uncategorized
In light of the recently published versions of USP chapters <795>, <797>, and <800>, with the intention of creating cohesive alignment between nonsterile and sterile compounding, it is essential to consider engineering controls for Hazardous Drug...
by Bryan Prince | Jan 16, 2021 | Uncategorized
Original Article Published Pharmacy Purchasing Products Magazine: Link to Full Article: Design a <795> Compliant Compounding Space : September 2019 – Pharmacy Purchasing & Products Magazine (pppmag.com) The purpose of USP General Chapter...

by Bryan Prince | Oct 6, 2020 | Uncategorized
(Transcript from Bryan Prince on The Pharmacy Inspection podcast – Listen to AUDIO here) Hello to all of my compounding friends and colleagues and welcome to another episode of the Pharmacy Inspection Podcast. Last week when Seth pulled me out of the...
by Bryan Prince | Sep 26, 2020 | Uncategorized
[Transcript from The Pharmacy Inspection Podcast- Episode 44 – Listen to Audio here.] There are quite a few “unintended consequences” with USP 800 compliance and unfortunately most of those are hitting the pocket books of pharmacies trying to decipher and adhere...






